



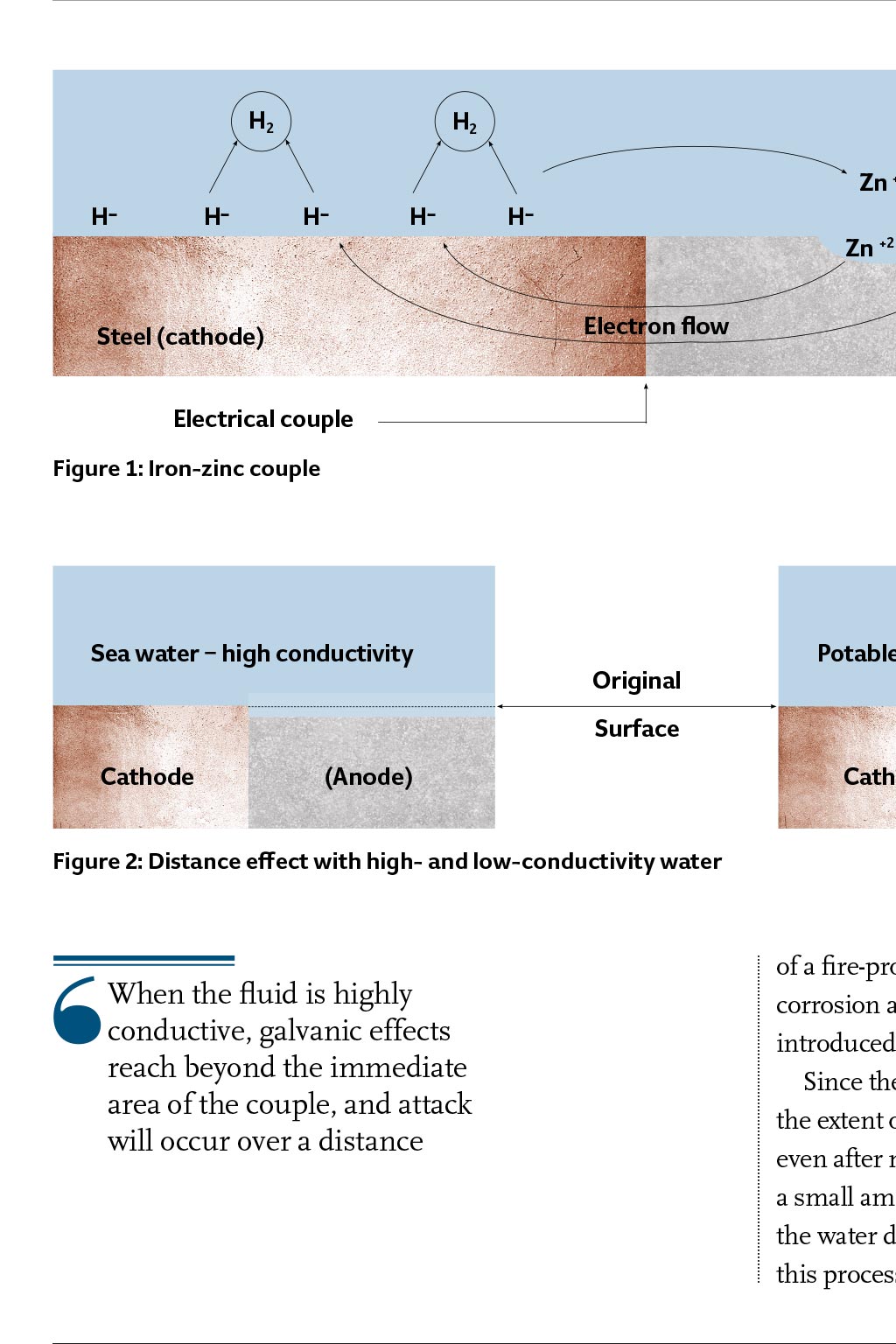
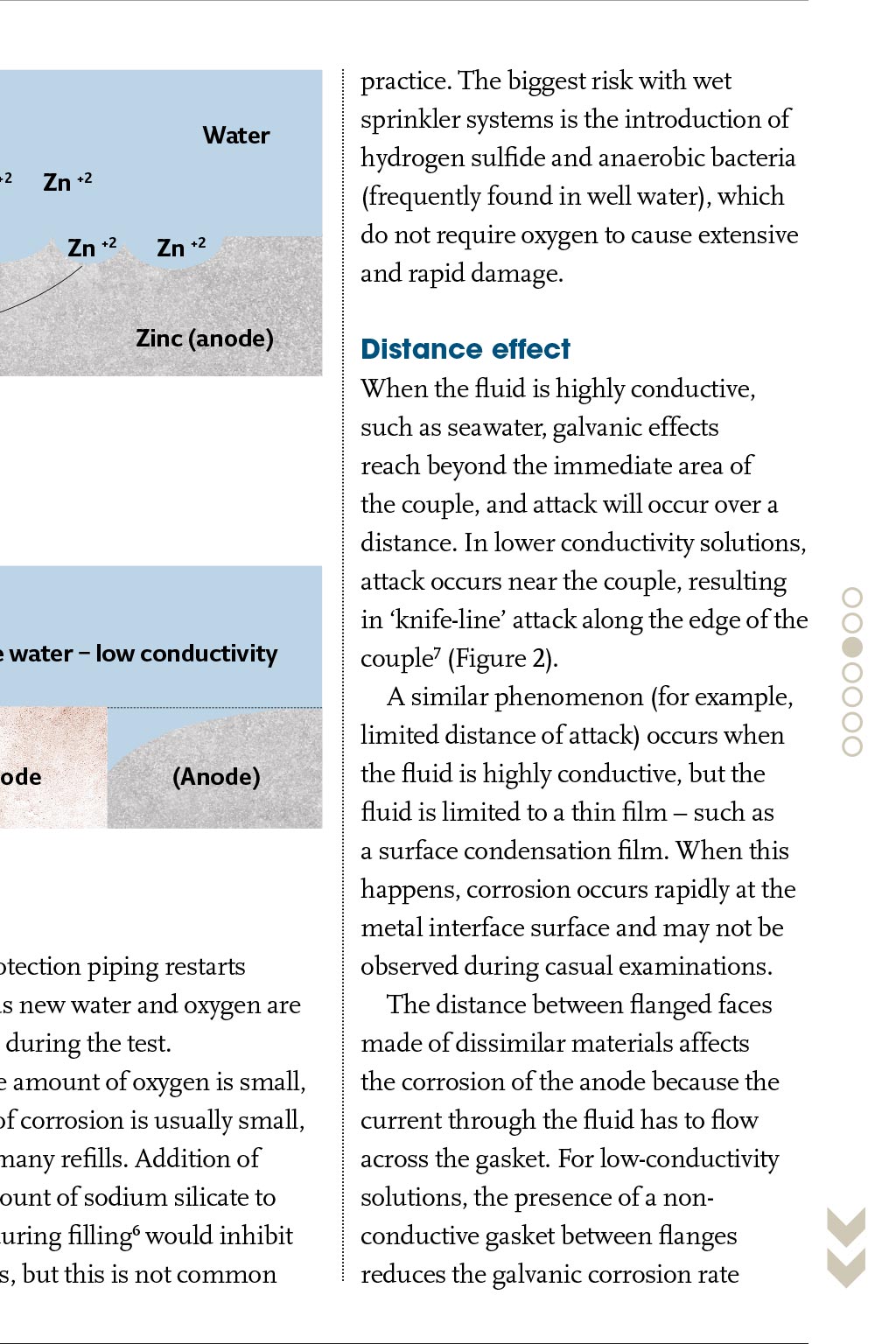

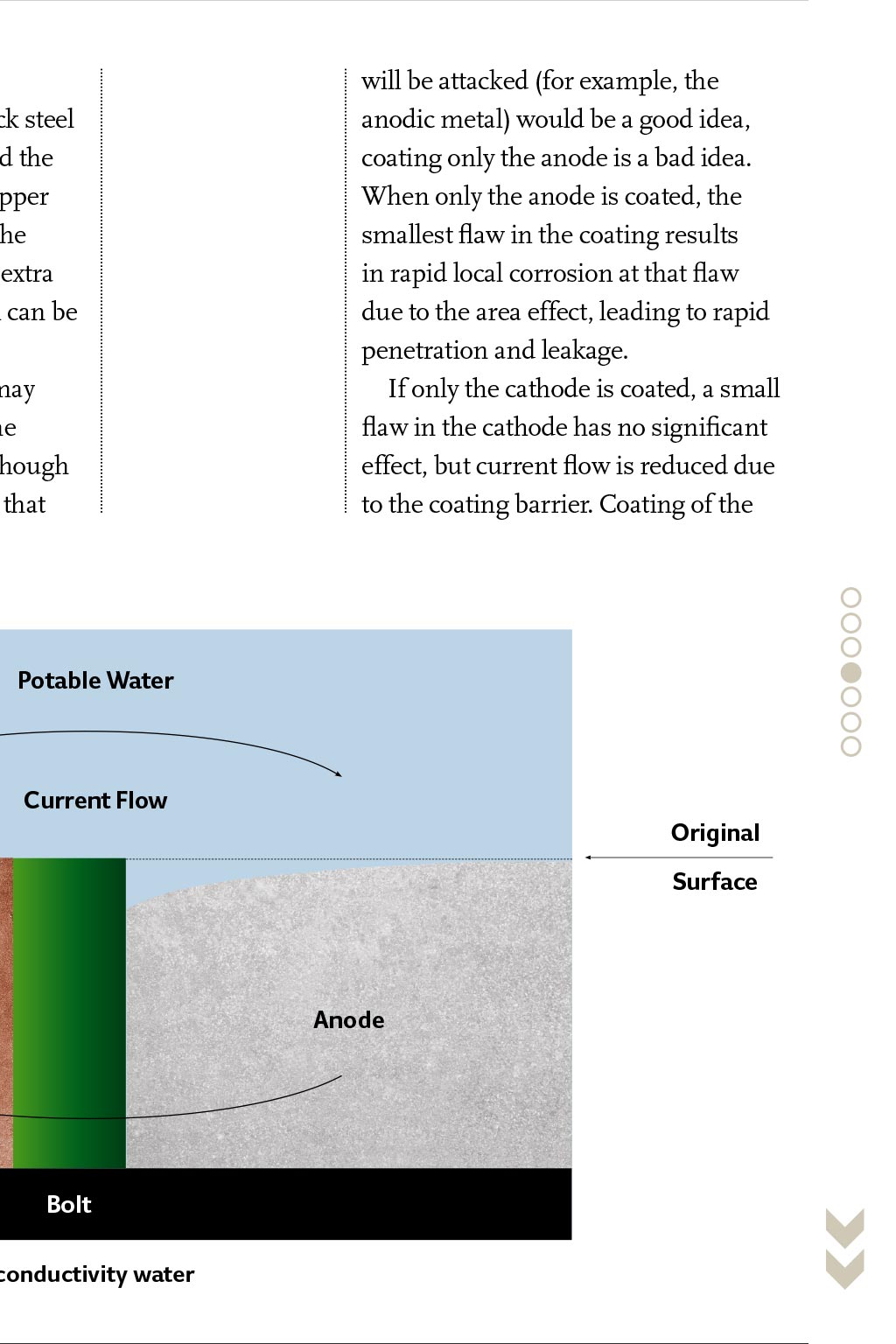



















ASHRAE GUIDANCE CORROSION DISSIMILAR M METALS HEATING AND AC PIPING SYSTEMS ost engineers understand that the presence of different metals in a heating or air conditioning piping system may cause accelerated local corrosion, and that one should avoid using dissimilar metals together wherever possible. This is good thinking, but sometimes availability or cost of materials make it necessary to join dissimilar metals together to build engineered piping systems. Using different metals does not mean that galvanic corrosion will cause a failure, nor does it mean that dielectric fittings must be used at transitions between materials. This article presents guidance on what to do when different Using different metals in heating or air conditioning metals are used in heating and airpiping does not necessarily mean corrosion will occur. conditioning piping systems. Walter J Sperko provides guidance on the successful use of dissimilar metals Reprinted by permission from ASHRAE Journal, April, 2009. Copyright. All rights reserved. and other high-chromium stainless steels, Inconels, copper-based alloys, tin, lead, cast iron and steel, aluminium and zinc. Magnesium is last and is the most anodic metal on the list. The further apart two metals are on a galvanic series, the greater the potential difference, and the faster the anode will corrode. Environmental effects The galvanic series, however, is not the final answer when determining the potential for galvanic corrosion. The arrangement of alloys in any galvanic series depends on the solution, and the specific corrosion behaviour of the metals involved in that solution. Three examples are: In Figure 1, current must flow between the metals and through the solution. If the fluid is nonconductive, galvanic corrosion does not occur. A zinc anode in a steel potable water tank will protect the steel because it is electronegative to the steel. However, if the water temperature exceeds 180F (80C), the reversal of potential occurs, and the steel tank becomes the anode and the zinc becomes the cathode.2 If this happens, the tank corrodes to protect the H2 H- H- Basics of aqueous corrosion Four conditions must be present for corrosion to occur in aqueous solutions: An anode A cathode A metallic current path between the anode and cathode An ionic conducting medium. Corrosion can be reduced by blocking zinc, which is not good. Thats why magnesium anodes should be used where the water temperature exceeds 160F (70C). When aluminium is coupled to steel, the aluminium acts as an anode in chloride solutions, such as brackish or seawater solutions, but it acts as a cathode in potable water.3 Fluid conductivity heavily influences the overall corrosion rate. Condensation that collects on the outside surface of a chilled water line that is indoors in an office/school/hospital environment is a comparatively low conductivity fluid. Accordingly, the corrosion rate due to condensation on the outer surface of a couple in such a line will be low. That same condensate on an outdoor line in an industrial or seacoast environment will be contaminated with salt or chemicals and become highly conductive; not only will a couple corrode at a greater rate, but the pipe will rust. Outdoor piping in industrial/seacoast environments should be protected even when there is insulation. Another factor that controls corrosion rate is the presence of corrosion products on the surface. These corrosion products form a barrier that slows the H- H- Zn +2 Zn +2 Zn +2 Electron flow Steel (cathode) practice. The biggest risk with wet sprinkler systems is the introduction of hydrogen sulfide and anaerobic bacteria (frequently found in well water), which do not require oxygen to cause extensive and rapid damage. Water H- Zn +2 Zinc (anode) Distance effect When the fluid is highly conductive, such as seawater, galvanic effects reach beyond the immediate area of the couple, and attack will occur over a distance. In lower conductivity solutions, attack occurs near the couple, resulting in knife-line attack along the edge of the couple7 (Figure 2). A similar phenomenon (for example, limited distance of attack) occurs when the fluid is highly conductive, but the fluid is limited to a thin film such as a surface condensation film. When this happens, corrosion occurs rapidly at the metal interface surface and may not be observed during casual examinations. The distance between flanged faces made of dissimilar materials affects the corrosion of the anode because the current through the fluid has to flow across the gasket. For low-conductivity solutions, the presence of a nonconductive gasket between flanges reduces the galvanic corrosion rate Electrical couple Figure 1: Iron-zinc couple Sea water high conductivity Original Potable water low conductivity Surface Cathode (Anode) Cathode (Anode) Figure 2: Distance effect with high- and low-conductivity water of a fire-protection piping restarts corrosion as new water and oxygen are introduced during the test. Since the amount of oxygen is small, the extent of corrosion is usually small, even after many refills. Addition of a small amount of sodium silicate to the water during filling6 would inhibit this process, but this is not common When the fluid is highly conductive, galvanic effects reach beyond the immediate area of the couple, and attack will occur over a distance because the current encounters more resistance due to the longer path it has to travel, as shown in Figure 3. For high-conductivity solutions, the gasket thickness makes no difference. Area effect Imagine that two copper plates are joined using steel rivets, and two steel plates are joined using copper rivets. Both are immersed in seawater. After 15 months, the steel rivets will have corroded away, but the copper rivets will still hold the steel plates firmly together.8 In both sets of plates, the steel is anodic and will corrode to protect the copper. Because the surface area of the copper plates is large compared to that of the steel rivets, the rivets will be consumed protecting the copper plates. Although the steel plates will rust, there is plenty of steel surface available to sacrifice itself to protect the copper rivets. In low-conductivity water, the overall corrosion rate is lower, and corrosion is limited to the steel immediately surrounding the copper rivets. Area effect is less of an influence with lowerconductivity solutions, but most lowconductivity solutions are less corrosive to begin with. cathode is usually an effective corrosionmitigation tool when couples are involved unless hydrogen evolution occurs at the cathode; when this happens, the coating will disbond and plug up the system. Coatings should be used with care. When evaluating the relative surface area of a couple, it is the wetted surface areas that affect corrosion. For example, if there are two different metals in a tank that may be partially filled at any time, the relative areas of the metals that are wetted at any given time control the corrosion rate at that time. That is, a carbon steel surge tank that has a stainless steel drain plug at the bottom wont suffer from much galvanic attack when the tank is full because there is a large wetted carbon steel (anode) surface. However, it may be attacked heavily when the tank is nearly empty because the wetted carbon steel (anode) surface is small. Inhibitors Corrosion inhibitors reduce the aggressiveness of the water. A properly inhibited closed-loop hot or cold system should last the lifetime of a building if its water and/or steam chemistry is maintained properly. treatment properly; this cannot be overemphasised. While maintaining the proper inhibitor levels in closed systems is easy, those systems should be monitored to ensure that they stay closed. Engineers should always install metering of makeup water in closed systems for three reasons: So that the rate of loss can be monitored and remedial action can be taken to stop unnecessary water losses. Potable water contains dissolved minerals and 6 to 12 milligrams of oxygen per liter (6 to 12 ppm), increasing the corrosiveness and scaling tendency of the water. Excessive makeup water leads to increased corrosion rates. Water treatment chemicals in the correct proportions must be added to makeup water to replace those that are lost to maintain the efficacy of the inhibitors. Makeup water should be checked ensure that contaminants are dealt with properly. Municipal water contains undesirable oxygen and chlorine. These can be eliminated by deaerating or chemically treating the water prior to pumping it into the system. Well water may not contain chlorine, but both metals should be used. The engineer should consult with a water treatment specialist to specify the inhibitor system to be used, and, the owner should be instructed on the importance of maintaining proper water chemistry For closed systems, the engineer should provide for metering of makeup water even though it may not seem necessary When insulating connections (dielectric couplings) are being considered, engineers should recognize that creep relaxation occurs in the plastic parts and gaskets that are standard. Specify insulating plastic grades that are suitable for the service temperature Dissimilar metals do not cause problems when the wetted surface area of the more noble metal (cathode) is small relative to the wetted surface area of the less noble metal (anode). Accordingly, stainless steel instrument connections, plugs, etc., in carbon steel piping tanks and similar will cause negligible local corrosion Threaded joints between dissimilar metals should be avoided unless corrosion inhibitors that protect both Galvanic couples The reason that joining two metals together may cause accelerated corrosion is that some metals are more easily corroded than others. This goes back to basic chemistry. All engineering metals react with their environment, and some are more reactive than others. The rate of reaction depends on the environment; when dissimilar metals are electrically connected to each other, each interacts with the environment, and the more reactive metal takes the brunt of the reaction, as shown in the iron/zinc couple in Figure 1. While corrosion in a couple always occurs at the anode, there is normally little if any corrosion at the cathode. The potential of metals for galvanically driven corrosion is ranked using the galvanic series, and the most widely published of these is for metals in seawater. At the top of this series1 (the most cathodic or corrosion-resistant) is platinum, followed by titanium, Hastelloys, 18-8 reaction rate and may effectively stop further attack. Titanium coupled to steel in highly conductive seawater, for example, does not result in accelerated corrosion of the steel, even though there is a large potential difference between titanium and steel. This is because the titanium forms an oxide barrier that blocks current flow.4 This phenomenon is referred to as polarisation. Some polarisation occurs with all metals when oxides and other corrosion products are formed on the surfaces; the extent of polarisation controls the rate of corrosion. As a result, each metal and each solution has its own electrochemical corrosion behaviour. Characteristically, corrosion rates are initially high, but they decrease as polarisation occurs. Polarisation occurs with individual metals and with couples. Wet fire protection systems are an interesting example of polarisation combined with a change in the environment. Wet fire protection systems are usually filled with potable water that is only mildly corrosive and contains some oxygen. The oxygen attacks the steel surface, forming a tight rust film. Once the oxygen is used up, corrosion stops5, including any corrosion associated with couples. Periodic testing H2 Zn +2 current access to the anode or cathode or both or by increasing resistance to current flow in the current path or the liquid medium. will be attacked (for example, the anodic metal) would be a good idea, coating only the anode is a bad idea. When only the anode is coated, the smallest flaw in the coating results in rapid local corrosion at that flaw due to the area effect, leading to rapid penetration and leakage. If only the cathode is coated, a small flaw in the cathode has no significant effect, but current flow is reduced due to the coating barrier. Coating of the Because of the area effect, thin copper tubes can be used in a thick steel tubesheet in a heat exchanger, and the steel tubesheet will protect the copper tubes from corrosion. Although the tubesheet will corrode over time, extra thickness of the inexpensive steel can be provided to compensate. The corrosion rate of couples may be reduced by coating or lining the members near the couple. Even though it seems that protecting the part that Potable Water Current Flow Original Surface Cathode Anode Bolt Figure 3: Effect of a gasket in low-conductivity water Inhibitors work by forming a thin film of oxides or organic compounds on metal surfaces that block the flow of current. When current flow is reduced, corrosion rates are reduced, including corrosion rates for dissimilar metal couples. There are two common metalbased inhibitors: molybdates and zinc. Molybdates form a complex oxide film with iron at the anode, whereas zinc will form a zinc hydroxide or carbonate film at cathodes. Both are usually used in conjunction with other inhibitors that will be described later. Chromates are effective corrosion inhibitors for steel, copper, and aluminum, but concerns over toxicity have largely eliminated their use. Non-metal inhibitors include phosphates, nitrites, nitrates, azoles, and silicates. Polyphosphates form a polarizing film on the cathodes, whereas orthophosphates combine with calcium to form an iron phosphate film on anodic surfaces of ferrous metals. Nitrites form their own film on anodic surfaces on steels and iron, whereas nitrates protect aluminum. Azoles are copper-specific inhibitors that support formation of a natural copper oxide film, and silicates will it can contain bacteria that can lead to microbiologically induced corrosion (MIC), and it can contain hydrogen sulfide (H2S), which is an aggressive chemical agent. Biological matter can be treated by filtering, which is followed by a biocide. H2S can be removed chemically or by steam deaeration. Inhibitors in open systems (for example, wet cooling towers) can work, but they must be supplied continuously, and biocides are also required. For openloop systems, cathodic protection of dissimilar metal couples by use of a third metal that is anodic to both metals or use of impressed current will stop general and galvanic corrosion and should be considered for large equipment. Dissimilar metal couples should be avoided in open-loop piping where cathodic protection of the inside of the pipe is impractical. Recommendations Heating and cooling piping system corrosion problems can be avoided by: Knowing what materials are in a system Knowing when they can cause a problem due to galvanic coupling and Selection of an appropriate system of water treatment metals are used. Brazing (with braze metal that is cathodic to both metals) or welding is preferred If coatings or liners are used, they should cover the cathodic metal in preference to both. Coatings should never be used only on the anodic metal When the outside of the joint may become wetted by condensation, rain, etc., the pipe that is installed outdoors should be protected by paint or similar coating even if insulated; this will also protect any dissimilar metal joints from corrosion For potable water systems, dielectric fittings should be used when connecting hot water tanks and heat exchangers and at-grade penetrations along with lightning protection to protect from external corrosion. The engineer should check for proper installation (gaskets, sleeves, washers, and o-rings) when dielectric fittings are specified. Gaskets should be suitable for the service temperature Conclusions Although different metals are used in a heating or air-conditioning piping system it does not mean that galvanic protect steel, lead, copper and copper alloys, and aluminum. Silicates are anodic inhibitors used with aluminum, particularly where coolants may contact food products. Other inhibitors are organic compounds such as amines9 that adsorb on the metal surface and suppress metal dissolution by blocking current flow into the fluid. Many organic inhibitors affect both the anodic and the cathodic reactions and can be used on steam and water systems. Oxygen corrodes metal, and reducing oxygen in a closed piping system reduces corrosion of the metals in that system. In neutral or slightly alkaline water, oxygen can be removed by using scavengers such as ascorbic acid or sodium sulfite,10 thereby reducing corrosion rates. Selection of an appropriate water treatment system is a task for a specialist. The engineer who designs the piping system should consult with a reputable water chemistry specialist to select a program of water treatment and corrosion inhibition that is suitable for the materials and temperatures in his system. The engineer should be sure that the eventual owner/operator of the piping system is cognizant of his responsibility to maintain the water The following apply when two different metals will be used: Galvanic corrosion does not occur when metals are dry. Systems handling noncondensing air, nitrogen, or other gases can have dissimilar metals joined together without concern for galvanic corrosion Galvanic corrosion is not a concern for families of similar metals because their electrochemical potentials in water are close to each other. The following families of materials are not subject to galvanic corrosion1 when materials within a family are coupled: Copper, Monel, copper-nickel, brass, and bronze alloys Steel, iron, and cast iron Aluminum alloys, although pure aluminum (1100 alloy) is anodic to other aluminum alloys Stainless steel, including 11% to 30% Cr alloys and austenitic stainless steels such as 304, 304L, 316, 316L; and Nickel and nickel alloys. All heating and air-conditioning piping systems handling water or steam should be protected from general corrosion by use of appropriate inhibitors. If dissimilar metals are in the system, an inhibitor system that protects corrosion will cause a failure or that dielectric couplings must be used. Engineers and owners should recognise that using dielectric isolators is not a substitute for proper water chemistry control, and that proper water chemistry control can eliminate the need for troublesome dielectric fittings, and also ensures a long service life for the piping system. CJ References: 1 Fontana, M G, 1986, Corrosion Engineering, NY: McGraw-Hill, p43. 2 Corrosion Engineering, p44. 3 Revie, R W, 2000, Uhligs Corrosion Handbook, Second Edition, John Wiley & Sons, p144. 4 Corrosion Engineering, p45. 5 Lane, R W, 1993, Control of Scale and Corrosion in Building Water Systems, NY: McGraw-Hill, p164. 6 Control of Scale and Corrosion in Building Water Systems, p203. 7 Uhligs Corrosion Handbook, Second Edition, p146. 8 Corrosion Engineering, p46. 9 Corrosion Engineering, p282. 10 Corrosion Engineering, p283. 11 Control of Scale and Corrosion in Building Water Systems, pp149-50. WALTER J SPERKO is president of Sperko Engineering Services